Abstract
[Purpose]
Rituximab combined with chemotherapy has been established as a standard of care for patients with newly diagnosed advanced stage follicular lymphoma (FL), especially with high tumor burden. However, the best chemotherapy partner of rituximab remains unclear. Owing to inevitable relapse and succumbing to histologic transformation (HT), there are prerequisites for first-line treatment to improve the outcome of FL patients. To elucidate the long-term outcome of FL patients more than 10 years after treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as first-line therapy in a prospective clinical trial, we report the 10-year follow-up results of the patients allocated to either R-CHOP arm in a randomized phase II/III trial (JCOG0203, NCT00147121), in which both arms of R-CHOP-21 and R-CHOP-14 showed almost equal survival.
[Patients and Methods]
Patients with untreated indolent B-cell lymphoma in advanced stages were assigned to the potential standard arm; six cycles of R-CHOP-21 or the experimental arm; R-CHOP-14 with G-CSF. Rituximab maintenance was not allowed (JCOG0203 Trial; Watanabe T, et al, J Clin Oncol 2011;29:3990). Histopathologic specimens from all 300 patients were reviewed by 3 hematopathologists. The primary endpoint of the study was progression-free survival (PFS). According to the planned analysis after 10 years following enrollment of the last patient, clinical event information was updated. PFS and overall survival (OS) were estimated by using the Kaplan-Meier method, and Hazard ratio (HR) and its confidence interval were estimated through the Cox proportional hazards model. The incidences of HT and secondary malignancies were analyzed by cumulative incidence function.
[Results]
From September 1st, 2002 to February 28, 2007, 300 patients were enrolled. The number of patients lost to follow-up within 10 years from randomization was 19 in all eligible patients. Ten-year PFS were 32.8% (R-CHOP-21) vs . 38.8% (R-CHOP-14), and 10-year OS were 80.9% vs . 84.7%, respectively in 299 eligible patients; namely, there were no differences. According to the Follicular Lymphoma International Prognostic Index, there were no differences found for either PFS or OS in any of the three risk groups.
We identified only male sex (HR: 1.57, 95% CI: 1.16-2.12) as a significant, unfavorable PFS factor, and age (>60) (HR: 2.03, 95% CI: 1.19-3.45) and increased LDH (>ULN) (HR: 2.38, 95% CI: 1.27-4.43) as significant, unfavorable factors of OS.
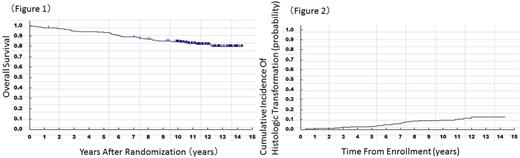
The proportion of FL was comparable between the arms (133 and 132 patients), and therefore, the combined analyses of the two arms were performed. In a total of 265 FL patients, 5, 8, and 10-year PFS were 46.2, 39.6, and 36.8%, respectively. Five, 8, and 10-year OS were 93.6, 86.7, and 84.4%, respectively (Figure 1).
HT was diagnosed in 27 of the 248 FL G1-3a patients (10.9% [95% CI: 7.3-15.5]), including 10 HT diagnosed clinically by the data including steep elevation of LDH levels or high accumulation of FDG (defined by SUVmax) in PET imaging (63.0% of HT was proved by biopsy). The pattern of the HT accumulation slope was similar to those reported previously but slightly different from that of PRIMA Trial, which showed more than half of them occurred within the first one year of follow-up; the cumulative incidence of HT at 3, 5, 8, and 10 years after enrollment were 2.4% (95% CI: 1.0-4.9), 3.2% (95% CI: 1.5-6.0), 8.5% (95% CI: 5.4-12.4), and 9.3 % (95% CI: 6.1-13.4), respectively (Figure 2).
The incidence of secondary malignancies in the 248 FL G1-3a patients at 10 years was 8.1% (95% CI: 5.1-12.0) [hematologic malignancies; 2.9%].
[Conclusions]
R-CHOP may reduce the incidence of HT compared with previous reports in the pre-rituximab era and did not increase a 10-year cumulative incidence of secondary malignancies in previously untreated FL patients, compared with published data. Although 10-year OS of advanced FL patients treated with R-CHOP without rituximab maintenance in the first-line setting was favorable, 10-year PFS was unsatisfactory. Dose-dense immunochemotherapy did not improve either PFS or OS in patients with indolent B-cell lymphoma. Therefore, the new treatments to deepen remissions and to prolong time to progression of FL patients are needed.
Tobinai: Chugai/Roche: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding; Zenyaku Kogyo: Honoraria. Kinoshita: Chugai: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Zenyaku: Honoraria, Research Funding; Jannsen: Honoraria; Kyowa Kirin: Honoraria; Bristol: Honoraria; Eisai: Honoraria; Takeda: Research Funding; Solasia: Research Funding; MSD: Research Funding. Yamaguchi: Eisai: Honoraria; Takeda Phamaceuticals: Honoraria; Kyowa Hakko Kirin: Honoraria; Chugai Pharma: Honoraria; Nippon Shinyaku: Honoraria. Ando: Kyowa Hakko Kirin: Research Funding; Takeda Pharmaceutical: Research Funding. Ogura: Astrazeneca: Honoraria; Takeda: Consultancy, Honoraria; Mundipharma: Consultancy; Celltrion, Inc: Consultancy, Honoraria; SymBio: Consultancy, Research Funding; Celgene: Consultancy, Honoraria; MeijiSeika Pharma: Consultancy. Tsukasaki: Chugai/Roche: Honoraria; Zenyaku Kogyo: Honoraria; Mundypharma: Research Funding; HUYA: Honoraria; Celgene: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Kyowa-Kirin: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.